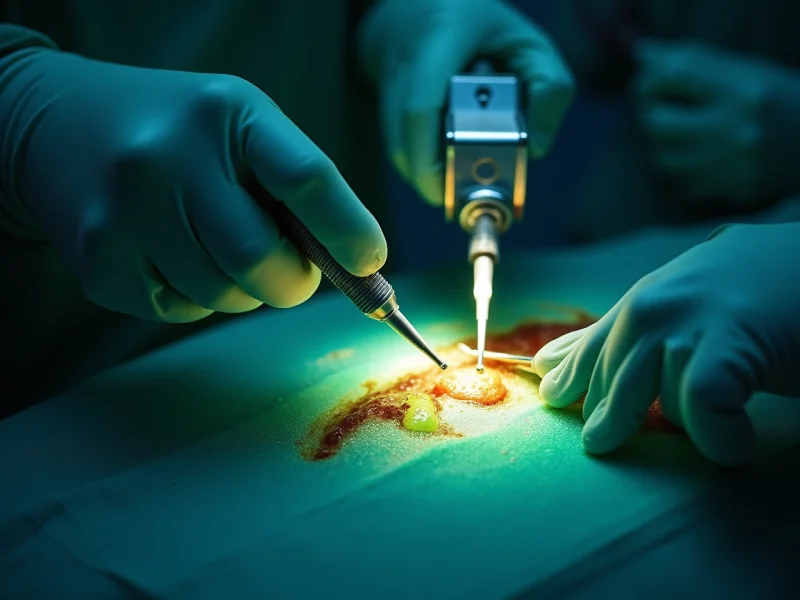
Firefly Imaging uses near‑infrared indocyanine green fluorescence integrated into the robotic camera to show real‑time tissue perfusion. It highlights vascularized areas as color overlays, helping surgeons identify poorly perfused segments and adjust resection margins or anastomoses. Clinical data link its use to fewer leaks, shorter stays, and reduced reoperations in colorectal and reconstructive procedures. Implementation requires team workflow changes and training for interpretation. More detail on mechanisms, outcomes, and practical steps follows.
Firefly imaging is an intraoperative fluorescence visualization system that enhances tissue contrast by detecting near-infrared light emitted from indocyanine green (ICG) dye administered to the patient. The system couples a near-infrared light source and camera with the robotic platform, converting invisible fluorescence into real-time color overlays on the surgeon’s console. Workflow involves intravenous ICG injection, brief circulation, and activation of the fluorescence technology mode to highlight vascularized structures against surrounding tissue. This surgical imaging tool augments normal white-light views without interrupting instrument control, offering fluid visualization of anatomy, lymphatics, and biliary structures. Interpretation requires awareness of timing, dose, and camera distance, as signal intensity reflects dye distribution rather than absolute tissue viability.
When assessing tissue perfusion intraoperatively, fluorescence imaging provides lively, real‑time visualization of blood flow by tracking the distribution of indocyanine green (ICG) within vessels and microvasculature. Surgeons interpret the intensity and timing of fluorescence to judge perfusion gradients, identify poorly perfused segments, and decide resection margins. Quantitative and qualitative signals correlate with tissue viability, aiding objective assessment beyond tactile or visual cues alone. The technique integrates seamlessly into robotic workflows, offering repeatable assessments after manipulation or reconstruction. Limitations include variable ICG dosing, camera settings, and patient factors that can affect signal interpretation; awareness of these variables is essential. Ongoing refinements in image processing and standardization aim to improve reproducibility and confidence in perfusion assessment.
Improved intraoperative visualization of perfusion has been associated with measurable reductions in anastomotic leaks and related complications in robotic surgery. Quantitative and qualitative fluorescence assessment helps surgeons identify poorly perfused segments before constructing anastomoses, directly supporting anastomotic integrity. Clinical series report fewer postoperative leaks, reduced reoperations, and shorter hospital stays when perfusion-guided decisions alter tissue selection or lead to reinforcement measures. Leak prevention is further aided by real-time feedback that can prompt additional suturing, diversion, or revised resection margins. While not a guarantee, this targeted approach refines intraoperative judgment, standardizes assessments across teams, and may reduce variability in outcomes. Ongoing studies aim to define thresholds and protocols to maximize the demonstrated clinical benefits.
Integrating the near-infrared fluorescence imaging system into robotic platforms requires coordinated hardware, software, and workflow alignment to guarantee seamless intraoperative use. The discussion addresses camera optics, light source modulation, and instrument compatibility to enable reliable visualization without disrupting console control. Software overlays and user interfaces present fluorescence data intuitively, supporting rapid interpretation while minimizing cognitive load. Compatibility with existing data streams and secure firmware updates facilitates consistent performance and regulatory compliance. Training modules and standardized protocols help translate firefly technology integration into routine practice, promoting team coordination between bedside and console surgeons. Ongoing collaboration between device manufacturers and clinical teams drives progressive improvements, reflecting broader robotic surgery advancements in safety, efficiency, and enhanced intraoperative imaging capability.
Clinical examples demonstrate how Firefly provides real-time perfusion assessment to inform tissue viability. Surgeons use this information to avoid ischemic margins and adjust resections accordingly. The technology also guides optimal anastomosis placement by highlighting well-perfused tissue during reconstruction.
Real-time perfusion assessment with Firefly imaging enables surgeons to visualize tissue blood flow intraoperatively, providing immediate feedback that can alter surgical strategy. Surgeons use real time imaging to compare perfusion across resection margins, anastomotic sites, and reconstructed flaps, guiding choices about tissue preservation or additional suturing. Intraoperative monitoring with fluorescence highlights subtle perfusion gradients invisible to standard white-light views, supporting decisions such as reinforcing anastomoses or trimming poorly perfused tissue. Case examples show reduced leak rates and fewer reoperations when teams act on Firefly data, and workflow integration allows rapid, repeatable assessments without significant operative delay. The modality complements clinical judgment, offering objective vascular maps that inform safer, more customized intraoperative choices.
Building on intraoperative perfusion mapping, Firefly imaging has repeatedly changed surgeons’ choices about resection margins to avoid ischemic tissue. Case examples show unexpected hypoperfused zones at planned transection lines, prompting immediate adjustment of margins and preservation of well-perfused tissue. The technology supports ischemia prevention by visualizing microvascular flow in real time, reducing reliance on tactile or visual estimates alone. In several reports, altering resections based on fluorescence eliminated areas at risk for necrosis and marginal breakdown. This improved intraoperative decision-making enhances surgical precision, shortens operative uncertainty, and may lower postoperative complications linked to ischemia. Collectively, these cases illustrate how objective perfusion data directly informs margin selection and optimizes tissue viability without prolonging the procedure.
Several reports demonstrate that Firefly imaging directly informs anastomosis placement by revealing perfusion gradients along bowel segments that are not evident to the naked eye. Surgeons adjust transection lines and choose more vascularized bowel for anastomosis optimization, reducing reliance on subjective visual cues. Case examples show intraoperative fluorescence applications identifying marginally perfused tissue that would otherwise be included in the anastomosis, prompting resection to healthier margins. In colorectal and small bowel procedures, this leads to altered operative plans, shorter ischemic segments, and more resilient anastomoses. Documentation of fluorescence patterns supports real-time decision-making and postoperative auditing. While not a guarantee against leaks, incorporating Firefly into workflow demonstrably refines anastomosis placement and supports risk-reduction strategies.
Recent clinical trials assessing perfusion imaging in robotic procedures have reported measurable improvements in surgical decision-making and patient outcomes. Several studies demonstrate an association between intraoperative fluorescence guidance and reduced complication rates, particularly fewer anastomotic leaks and reoperations. These findings support further integration of perfusion imaging into practice while highlighting the need for larger randomized trials to confirm long-term benefits.
Clinical trial outcomes for indocyanine green (ICG)-based perfusion imaging in robotic surgery provide focused evidence on safety, diagnostic accuracy, and clinical impact across multiple specialties. Trials with varied clinical trial design—randomized, prospective cohort, and observational—have evaluated perfusion visualization in colorectal, urologic, and reconstructive procedures. Endpoints commonly include real-time assessment concordance with clinical judgment, reproducibility of fluorescence patterns, and correlations with short-term patient outcomes such as wound healing and graft viability. Reported safety profiles show low adverse event rates related to ICG. Studies document improved intraoperative decision confidence and altered surgical plans when fluorescence data indicate compromised perfusion. Limitations noted include heterogeneous protocols, small sample sizes, and the need for standardized quantitative thresholds to strengthen cross-study comparisons and guideline development.
Building on evidence that ICG-based fluorescence alters intraoperative decisions and is safe across specialties, multiple studies have specifically examined whether perfusion imaging reduces postoperative complications. Meta-analyses and prospective cohorts report lower rates of anastomotic leak, tissue necrosis, and reoperation when intraoperative fluorescence guides resections and reconstructions. Reduced complication rates translate into improved surgical success metrics—fewer readmissions, shorter length of stay, and decreased need for revision procedures. Benefits appear most pronounced in colorectal, bariatric, and reconstructive cases, where marginal perfusion is common. While randomized data remain limited, consistent observational signals support incorporation of perfusion imaging as a tool to enhance patient safety. Ongoing trials aim to quantify absolute risk reductions and cost-effectiveness across varied surgical populations.
When integrated into the operating room, Firefly fluorescence imaging requires coordinated adjustments to team roles, equipment placement, and procedural timing to guarantee reliable visualization without disrupting the surgical flow. The team assigns specific responsibilities: an anesthesiologist times indocyanine green injections, a bedside assistant manages camera toggles, and the console surgeon interprets live images. Clear team communication protocols and briefing checklists preserve operational efficiency and reduce delays. Equipment placement prioritizes unobstructed camera lines and easy access to contrast agents while minimizing cable hazards. Procedural timing aligns imaging moments with critical decision points to avoid repeated dosing. Documentation of imaging findings in the operative record and brief post-case debriefs reinforce continuous improvement and workflow refinement.
Although proficiency in fluorescence interpretation depends on repeated exposure, structured training accelerates the acquisition of reliable pattern recognition and decision-making. Simulation modules, case-based review, and proctored live cases form a curriculum that teaches signal interpretation techniques, threshold recognition, and common artefact identification. Trainees learn standardized image acquisition, timing of dye administration, and correlation of fluorescence patterns with clinical endpoints. Assessment uses objective metrics: accuracy in border delineation, inter-rater reliability, and time to decision. Fluorescence training programs integrate didactics on physics and pharmacokinetics with hands-on practice using recorded and simulated scenarios. Ongoing competency is maintained through periodic refreshers and outcome feedback loops so that surgeons apply fluorescence data consistently and minimize variability in intraoperative judgment.
Even modest reductions in reoperation rates attributable to intraoperative fluorescence guidance can translate into substantial cost savings for health systems, driven by lower operating-room utilization, shorter total hospital stays, and fewer postoperative complications requiring intervention. A focused cost analysis compares the incremental expense of Firefly-capable robotic systems and indocyanine green against avoided costs from fewer returns to the OR, reduced intensive-care days, and diminished readmission penalties. Financial benefits accrue not only from direct savings on procedures but also from improved throughput, optimized bed use, and reduced staffing strain. When modeled across typical annual caseloads, modest percentage decreases in reoperations yield net savings that often justify initial investment, particularly in high-volume centers where marginal gains compound.
As fluorescence-guided robotic surgery matures, research and development are converging on three linked priorities: enhancing probe specificity and sensor sensitivity, integrating real-time quantitative analytics into the surgical console, and expanding tracer portfolios for varied tissue types and pathologies. Future innovations will emphasize molecular-targeted fluorophores, multispectral detectors, and miniaturized probes to improve signal-to-noise and lesion discrimination. Technological advancements in machine learning and augmented reality aim to deliver live perfusion metrics, predictive leak risk scores, and guidance overlays without distracting workflow. Regulatory pathways and standardized validation studies are needed to translate prototypes into clinical tools. Cost-effectiveness analyses and training curricula will shape adoption, ensuring that emerging fluorescence capabilities meaningfully improve decision-making, reduce complications, and broaden applications across specialties.
At Dr. Brian Harkins, we are committed to enhancing robotic surgery through our innovative Firefly imaging technology. This advanced system provides real-time fluorescence visualization of tissue perfusion, empowering our surgeons to make informed intraoperative decisions that significantly reduce anastomotic leaks and associated complications. By seamlessly integrating Firefly imaging into robotic platforms, we streamline workflow, support targeted training, and potentially lower costs by minimizing the need for reoperations. Our case experiences have consistently demonstrated improved tissue assessment and better patient outcomes. We are dedicated to continuing technological and clinical research to refine our fluorescence-guided techniques, expanding their applications and solidifying Firefly’s pivotal role in making minimally invasive surgery safer and more efficient.
The Da Vinci Surgical System by Intuitive Surgical represents a groundbreaking advance in minimally invasive procedures, allowing surgeons to perform robotic-assisted and laparoscopic surgery with enhanced precision. Compared to open surgery, this surgical system provides superior dexterity, 3D visualization, and reduced operative time, especially in colorectal surgery, thoracic surgery, and urologic surgery.
The Firefly System, integrated into the Da Vinci Surgical System, enables near-infrared fluorescence imaging with indocyanine to visualize real-time blood flow and tissue perfusion. The use of indocyanine green (ICG) dye allows intraoperative assessment of vascular structures, guiding safer surgical procedures and supporting accurate visual assessment of vessels during fluorescence-guided surgery.
Indocyanine green fluorescence imaging provides dynamic feedback on colorectal anastomotic perfusion, improving outcomes in patients undergoing robotic colorectal or robotic low anterior resection. The use of ICG facilitates anastomotic perfusion mapping, reducing leak rates and ensuring adequate oxygenation of the rectal stump compared to the control group in clinical studies.
Fluorescence in robotic general surgery leverages near-infrared imaging with indocyanine green to enhance visualization of anatomy within the surgical field. Surgeons can perform minimally invasive surgery using standard endoscopic instruments while benefiting from fluorescence imaging of biliary ducts, especially during robotic cholecystectomy or cancer surgery, reducing bile duct injuries and improving precision.
The application of fluorescence through ICG fluorescence imaging aids use of perfusion assessment in real time. This enables the intraoperative fluorescence imaging of tissues, supporting accurate visual assessment and timely decision-making in surgery using Firefly or other fluorescence in robotic systems. It’s particularly useful in evaluating perfusion in patients undergoing colorectal surgery or rectal cancer resections.
Surgery using Firefly integrates green fluorescent visualization via injection of indocyanine green, offering enhanced tissue differentiation that laparoscopic and invasive surgery using standard endoscopic cameras cannot achieve. This fluorescence in robotic visualization ensures better margin control during tumor excision, facilitating improved oncologic outcomes in colorectal, thoracic, and urologic procedures.
In rectal cancer operations, fluorescence imaging with indocyanine green allows for precise localization of the lymph node basin and reliable intraoperative assessment of rectal stump perfusion. This technology supports fluorescence-guided surgery, enabling accurate dissections and better oncologic clearance in robotic-assisted or minimally invasive colorectal surgery.
When using the Da Vinci platform, surgical technique and perioperative outcomes are optimized through steady instrument control and stable visualization. Surgeons can perform minimally invasive surgery using the Da Vinci Surgical platform while integrating use of Firefly for vascular mapping. Compared with traditional general surgery, robotic assisted approaches reduce blood loss and enhance postoperative recovery.
The current status of fluorescence in robotic-assisted and robotic assisted oncology is promising. Studies show that using fluorescence with indocyanine green fluorescent imaging improves visual assessment and use of perfusion assessment across colorectal, urologic, and thoracic surgery. Ongoing review of the literature confirms significant safety advantages in tumor localization and margin verification.
Future surgical systems will rely heavily on near infrared and indocyanine green fluorescent innovations to guide surgery using standard endoscopic visible imaging. Using the Firefly System or similar platforms, imaging with indocyanine green will refine visual assessment and anastomotic perfusion, helping surgeons treat complex cases among 40 patients undergoing robotic and minimally invasive surgery using standard protocols.

Dr. Brian Harkins is a renowned surgeon specializing in advanced, minimally invasive, and robotic surgical techniques. With a dedication to innovation and personalized patient care, he has transformed countless lives by delivering exceptional outcomes.
I want a website like this, where do i start?